Abstract
Power modulation in an atmospheric pressure capacitively coupled radio frequency plasma jet is investigated by numerical modelling. The dynamics of successively pulsing the applied power on and off for a helium–oxygen (∼0.6%) plasma is investigated. The impact of power pulsing on reactive species generation and gas heating is discussed with control opportunities emphasized. Power modulation shows linear control for reactive species and heat flux delivery to a treatment surface above an initial phase of power growth. Power is found to be coupled primarily to the electrons with electron loss rates determining the interference between successive power modulation phases. Plasma decay in the power off phase is characterized by a large initial electron loss in the first 0.5 µs followed by ambipolar decay dominated by ions of opposite charge. Power modulation effects on gas heating show a larger range of temperature control when compared with convection cooling. Reactive oxygen species reaching a treatment surface are shown to typically vary over an order of magnitude for variation in the duty cycle.
Export citation and abstract BibTeX RIS
1. Introduction
Control of reactive species and heat flux is a key challenge for emerging applications of low temperature atmospheric pressure plasma technology in biomedicine [6, 9, 11, 14, 15, 20, 47]. Treatment of living organisms brings the requirement of precise dosage which often dictates the balance between therapeutic and toxic effects. Limitation of heat flux is critical in the uptake of plasma technologies on treatment surfaces sensitive to thermal shock. In the context of biomedical applications, for example, plasma induced temperatures above normal body temperatures of ∼40 °C for prolonged periods could damage cells and tissues not targeted by a particular plasma therapy. Continuously powered radio frequency (RF) plasma jets such as the micro-Atmospheric Pressure Plasma Jet (µAPPJ) [46] operate in a complex parameter space dependent on the operating power, O2% admixture to the helium jet, gas flow, device to surface separation and jet orientation [7, 8, 31, 32, 37]. The electro-negative nature of these discharges results in a non-linear power coupling for variation in O2 admixture [18]. Delivery of reactive species such as atomic oxygen and ozone to treatment surfaces depends on the gas dynamics in the discharge and effluent [18]. This is highly sensitive to the distance between the source and the surface due to the mixing of the jet effluent with the surrounding air [8]. Modulation of the applied power offers a potentially useful mechanism for control of reactive species and heat flux to treatment surfaces which is explored in this report.
The µAPPJ [46] device has emerged as a benchmark source for numerical [13, 16, 26, 30, 31, 38, 48] and experimental investigation [7, 8, 31, 32] of RF plasma jets for development of applications in biomedicine and heat sensitive surface engineering. The µAPPJ consists of two parallel steel electrodes of 1 mm thickness and 30 mm in length surrounded on two sides by 1 mm thick transparent quartz allowing optical diagnosis of the plasma core. Small admixtures of atmospheric gases such as O2 can be added to a helium carrier gas to generate reactive oxygen species (ROS) with additional nitrogen contributions from impurities in helium and the surrounding air resulting in reactive oxygen nitrogen species (RONS) production. Reports on experimental [39, 40] and numerical studies [22, 45] of RF capacitively coupled plasma (CCP) jets have discussed the effect of power modulation on electro-positive gas mixtures in helium. This report presents numerical evidence for behaviour in an electro-negative gas mixture of helium and oxygen (∼0.6%). Power modulation effects on plasma growth and decay, reactive species generation and gas heating are discussed. The effects of duty cycle variation on ROS generation and gas heating are investigated in this context. Comparison of our numerical results for a continuously powered jet with molecular beam mass spectrometry measurements [8] can be found in [19].
2. Model description
The commercial finite element partial differential equation solver COMSOL Multiphysics (version 4.3a) [28] is used in this work. A sinusoidal applied voltage Vapplied sin (2π ft) with an rf frequency of f = 13.56 MHz is considered where Vapplied is the applied voltage amplitude. A frequency of 100 kHz is used over a range of duty cycles to modulate the applied power.
The µAPPJ [46] geometry consists of a canonical capacitively coupled discharge setup with parallel stainless steel electrodes 30 mm in length operated at electrode gaps of 0.5–2 mm. A plasma is generated in domain GHIL between two steel electrodes (EFGH & JKLI) shown in figure 1. For the µAPPJ device considered here the electrodes shown in figure 1 are placed between a quartz encasement to contain the gas flow. This feature is not considered in this geometry similar to earlier computational reports [13, 16] in order to construct a computational tractable model. Gas dynamics and mixing are therefore constrained to a 2D cross section across the electrodes. This assumption is increasingly valid for µAPPJ geometries where the thickness of the encasement perpendicular to the plane of electrodes shown in figure 1 is substantially larger than the width of the electrodes. For 'thin' encasements three dimensional flow descriptions may be more accurate. We restrict ourselves to the former scenario in this report.
Figure 1. Model geometry: µAPPJ [46].
Download figure:
Standard image High-resolution imageThe popular fluid model approach with a drift-diffusion approximation for description of the plasma dynamics is used here. Solutions of continuity equations for the electron density, electron energy and heavy species densities are coupled with solutions of Poisson's equation for the electric field over a horizontal 1D cross section across the electrode gap (HILG in figure 1). This 1D plasma model approximates the plasma dynamics as homogeneous across the discharge region HILG shown in figure 1. Reactive neutral species production and gas heating rates are extrapolated from solutions of the 1D plasma model across the 2D electrode domain for use as source terms in a 2D model of a reacting, mixing and heating gas of uncharged species convecting through the electrodes onto a substrate below the device. Decoupling of plasma dynamics from the gas and heat dynamics in this manner allows tractable solutions for the study of uncharged RONS and gas heating over longer time-scales. Such a decoupling relies on the assumption that the charged and neutral species produced by the plasma are weakly interacting. The plasma density (∼1017 m−3) in this context is typically several orders of magnitude lower than the steady state densities of O, O2(a 1Δ) and O3 (∼1021 m−3) [16, 24, 48]. Uncharged plasma produced species density are therefore not significantly effected by losses due to interaction with charged species. Charged and excited species densities in the plasma may however be changed remarkably by the influence of plasma produced ROS. Niemi et al [31] showed that the helium meta-stables are predominately quenched by penning ionization with O2 over O2(a 1Δ), O3 and O interaction. Charge transfer effects due to O2(a 1Δ), O3 and O interaction can change the composition of negative charge carriers depending on the O2 admixture [24] but are assumed here not to lead to large inaccuracies in the overall electrical behaviour of the plasma given the similar reaction pathways and transport properties of these negative charge carriers [24, 29]. Electron attachment for negative charge carriers with atomic oxygen is however included here (see below) due to its importance in the decaying plasma.
Gas dynamics are studied by solution of mass and momentum continuity equations (compressible Navier–Stokes). A mass transport convection–diffusion formulation is coupled to the Navier–Stokes equations to study the transport and interaction of plasma produced reactive neutral (uncharged) species. A thermal energy equation in the gas and solid phase is solved for the temperature of the gas mixture and electrodes. Thermal conductivity, material density and specific heat capacity values at constant pressure for steel electrodes is taken as 44.5 W (m K)−1, 7850 kg m−3 and 475 J (kg K)−1 respectively here. Temperature dependent values for thermal conductivity and specific heat capacity for the gas mixture are used [27]. Maximum pressure variations were measured as ∼1% of atmospheric pressure for the flow conditions used in this report. The constant pressure assumption for specific heat capacity used here therefore does not lead to significant error as heat is added to our system at approximately constant pressure. Further details of equation formulation, boundary conditions, transport properties and discussion are available in [18, 19, 35].
The plasma chemistry consists of six ionic species (He+,
 ,
,
 ,
,
 , O− and
, O− and
 with thirteen neutral species (He, He*,
with thirteen neutral species (He, He*,
 , O2, O2(a 1Δ), O, O (1D), N2, N2(A 3Σ), N2(B 3Π), N, N (2D)). Plasma produced neutral species (O, O2(a 1Δ), O (1D), N..) react to form ozone O3, various nitrogen–oxygen species NxOx (NO, NO2..), hydrogen–oxygen species HxOx (OH, H2O2...) and hydrogen–oxygen–nitrogen HNOx species (HNO2, HNO3..) [18]. Short lived reactive neutrals such as He*, H, O(1D) N(2D), N2(A 3Σ) and N2(B 3Π) formed during the plasma dynamics are not considered to convect or diffuse out of the discharge region. A helium purity of 99.999% is assumed here (10−5 air fraction) with the impurity considered to be made up of 20% O2 and 80% N2. The helium–oxygen plasma chemistry is shown in table 1. Electron detachment reactions R24 and R25 included in table 1 are dependent on the atomic oxygen density. Steady state atomic oxygen density develops on much larger time-scales (∼0.1 s) compared with the smaller (∼µs) time-scale of charged species behaviour. In order to approximate the atomic oxygen density a fixed value based on O2 depletion rates of 0.3% for conversion of O2 to O is used here. This depletion rate is consistent with measured [8] atomic oxygen density (∼1021 m−3) values for the µAPPJ source. Air impurities in the helium carrier gas are assumed to be made up of 79% N2, 20% O2 and 1% H2O. Electron transport and electron impact reaction rates are preprocessed by solving the zero dimensional Boltzmann equation using the Bolsig+ solver software [12, 41] coupled with collision cross section data from the LXcat database [25] for a range of helium–oxygen mixtures. Further details of the helium–nitrogen plasma chemistry and the uncharged species chemistry employed here can be found in [18].
, O2, O2(a 1Δ), O, O (1D), N2, N2(A 3Σ), N2(B 3Π), N, N (2D)). Plasma produced neutral species (O, O2(a 1Δ), O (1D), N..) react to form ozone O3, various nitrogen–oxygen species NxOx (NO, NO2..), hydrogen–oxygen species HxOx (OH, H2O2...) and hydrogen–oxygen–nitrogen HNOx species (HNO2, HNO3..) [18]. Short lived reactive neutrals such as He*, H, O(1D) N(2D), N2(A 3Σ) and N2(B 3Π) formed during the plasma dynamics are not considered to convect or diffuse out of the discharge region. A helium purity of 99.999% is assumed here (10−5 air fraction) with the impurity considered to be made up of 20% O2 and 80% N2. The helium–oxygen plasma chemistry is shown in table 1. Electron detachment reactions R24 and R25 included in table 1 are dependent on the atomic oxygen density. Steady state atomic oxygen density develops on much larger time-scales (∼0.1 s) compared with the smaller (∼µs) time-scale of charged species behaviour. In order to approximate the atomic oxygen density a fixed value based on O2 depletion rates of 0.3% for conversion of O2 to O is used here. This depletion rate is consistent with measured [8] atomic oxygen density (∼1021 m−3) values for the µAPPJ source. Air impurities in the helium carrier gas are assumed to be made up of 79% N2, 20% O2 and 1% H2O. Electron transport and electron impact reaction rates are preprocessed by solving the zero dimensional Boltzmann equation using the Bolsig+ solver software [12, 41] coupled with collision cross section data from the LXcat database [25] for a range of helium–oxygen mixtures. Further details of the helium–nitrogen plasma chemistry and the uncharged species chemistry employed here can be found in [18].
Table 1. He–O2 plasma chemistry: Notes: (1) Ri(n) - n indicates reference for ith reaction (2) Rates in units [m3 s−1], [m6 s−1] (3 body reactions), Tg (K) gas temperature, Te (K) electron temperature except where stated otherwise (3) M represents background gases He, N2, O2.
| Ref | Reaction | Rate |
|---|---|---|
| R1 [3, 41] | e + He → He + e | BOLSIG+ |
| R2 [3, 41] | e + He → He* + e | BOLSIG+ |
| R3 [3, 41] | e + He → 2e + He+ | BOLSIG+ |
| R4 [44] | e + He* → e + He | 2.9 × 10−15 |
| R5 [43] | e +
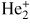 → He* + He → He* + He |
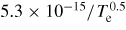 |
| R6 [10] | He+ + 2He → He +
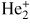 |
1.1 × 10−43 |
| R7 [10] | He* + 2He → He +
 |
2 × 10−46 |
| R8 [10] | He* + He* → e +
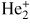 |
1.5 × 10−15 |
| R9 [10] |
 → 2He → 2He |
104 |
| R10 [10] |
 + +
 → e + → e +
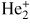 + 2He + 2He |
1.5 × 10−15 |
| R11 [3, 41] | e + O2 → 2e +
 |
BOLSIG+ |
| R12 [4, 41] | e + O2 → e + 2O | BOLSIG+ |
| R13 [4, 41] | e + O2 → e + O + O (1D) | BOLSIG+ |
| R14 [21] | e +
 → 2O → 2O |
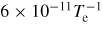 |
| R15 [42] | He* + O2 → He +
 + e + e |
2.54 × 10−16 (Tg/300)0.5 |
| R16 [2] |
 + O2 → 2He + + O2 → 2He +
 + e + e |
1 × 10−16 (Tg/300)0.5 |
| R17 [3, 41] | e + O2 → O + O− | BOLSIG+ |
| R18 [29] | e + He + O2 →
 + He + He |
3.6 × 10−43 Te[eV]−0.5 |
| R19 [21] | O− +
 → O + O2 → O + O2 |
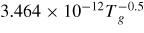 |
| R20 [21] |
 + +
 → O + O2 → O + O2 |
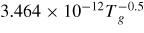 |
| R21 [21] | O− +
 + He → O + O2 + He + He → O + O2 + He |
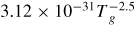 |
| R22 [21] |
 + +
 + He → 2O2 + He + He → 2O2 + He |
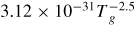 |
| R23 [29] | O− + O2 →
 + O + O |
1.5 × 10−18 |
| R24 [42] | O− + O → O2 + e |
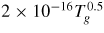 |
| R25 [42] |
 + O → O3 + e + O → O3 + e |
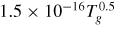 |
| R26 [3, 41] | e + O2 → e + O2(a1Δ) | BOLSIG+ |
Average variation in power, heating and species density with pulse modulation are investigated by solving an additional time dependent variable given by the following
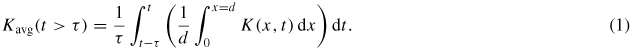
The applied voltage period is given by τ (s) here. The variable x represents the spatial variation for Kavg between the electrodes for the 1D plasma model. The length d (m) represents the electrode separation. Kavg(t) represents a continuous time average over the interval [t − τ, t] for the average over the discharge region ([0,d]) of the model variable K(x, t).
The partial differential equations discussed above are solved for a weak formulation using a finite element discretion [33] with the time dependent solvers available in COMSOL Multiphysics (version 4.3a) [28]. Lagrange linear elements are chosen for basis functions with minimum mesh elements ∼µm. COMSOL implements a time-dependent solver algorithm called implicit differential algebraic (IDA) [1] which uses a variable order variable step-size backward differentiation formula (BDF) method for integration of the time integral. BDF is a linear multi-step method that approximates the derivative of the function using previous time step information. The BDF method is a fully implicit method and requires the solution of a set of non-linear equations at each time step carried out using a Newton solver [5]. Initially the Newton solver finds the solution of a linear system using the PARDISO linear solver algorithm [34, 36]. The Newton iteration then continues by reducing a damping factor until the error reaches a specified tolerance or a minimum damping factor is exceeded.
3. Results and discussion
Results of the 1D and 2D numerical models for a µAPPJ helium/oxygen (0.6%) plasma jet powered with voltage amplitude of 325 V, a frequency of 13.56 MHz and modulated by a frequency of 100 kHz are discussed here. The duty cycle of the power pulse width lasting 10 µs is varied to investigate the interaction of successive pulses focusing on the effects of gas heating and reactive species generation. Plasma power and species density behaviour is discussed in section 3.1. Gas heating by the plasma and the resulting gas temperatures impacting a treatment surface is discussed in section 3.2. Production rates of reactive species due to power modulation and resultant species density at the treatment surface are discussed in section 3.3. Comparison of our numerical results for a continuously powered jet with molecular beam mass spectrometry measurements [8] can be found in [19].
3.1. Power modulated plasma behaviour
The average power (Pavg(t)) deposited to the plasma is shown in figure 2 for a range of duty cycles. An initial period of exponential growth is bounded by asymptotic convergence to a steady state power (ΔPavg < 5%) beyond a 30% duty cycle. Changes in duty cycles between 20–90% shown in figure 2 do not affect substantially the length of this power growth phase. This demonstrates the weak interference between neighbouring power pulses for duty cycles up to 90% observed. In the following discussion we will see how this behaviour is linked to the large electron decay in the first 0.5 µs during the power off phase.
Figure 2. Power Pavg(t) W m−3 (continuous phase and volume averaged) for a range of duty cycles over a 100 kHz modulation period.
Download figure:
Standard image High-resolution imageThe majority of the power is found to be coupled to electrons here with average values of 86.3% of the steady state power of 1.71 W m−3. The remaining contribution is dominated by power coupling to negative ions accounting for 13.4%. Figure 2 shows that increasing the duty cycle above 20–30% increases the period of power sustained at the steady state power. The average (over modulation period) plasma power varies here from 14.1% of the continuous power at a 20% duty cycle to 88.7% at a 90% duty cycle.
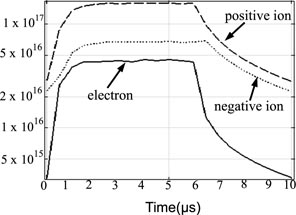
The electron, total positive and negative ion density are shown in figure 3 for a 60% duty cycle (6 µs power-on, 4 µs power-off). The electron density increases initially from a background value of ∼3 × 1015 m−3 of residual electrons from the previous power cycle. Steady state densities are reached after 20–30% of the modulation cycle (2–3 µs) corresponding to 27–41 cycles of the applied voltage (13.56 MHz). The average electro-negativity is 1.6 in the power-on phase (0–6 µs). O− and
 negative ion species have an average density of ∼3.7 × 1016 m−3 and ∼2.2 × 1016 m−3 respectively.
negative ion species have an average density of ∼3.7 × 1016 m−3 and ∼2.2 × 1016 m−3 respectively.
 is the dominant positive ion with average density of 1.4 × 1017 m−3. Mean electron energy values of 0.26 eV occurring at 2 µs increase to 4.03 eV at 6 µs. Average values for the power-on (0–6 µs) phase were 3.8 eV here. During the power-off phase average energy values drop to 0.08 eV at the end of the modulation period (10 µs).
is the dominant positive ion with average density of 1.4 × 1017 m−3. Mean electron energy values of 0.26 eV occurring at 2 µs increase to 4.03 eV at 6 µs. Average values for the power-on (0–6 µs) phase were 3.8 eV here. During the power-off phase average energy values drop to 0.08 eV at the end of the modulation period (10 µs).
Figure 3. Average (continuous phase and volume averaged) charged species density (1 m−3) behaviour over a modulation period for a 60% duty cycle.
Download figure:
Standard image High-resolution imageFigure 4 shows the electron density behaviour for a range of duty cycles. Electron density growth, which determines the degree to which consecutive modulation periods interfere, shows similar behaviour to power growth shown in figure 2. An increase in duty cycle from 20 to 90% results in an increase of the initial average electron density from 1.9 × 1015 m−3 to 8.3 × 1015 m−3. This initial residual electron density corresponds to 4.1% and 18.7% respectively of the average electron density of 4.5 × 1016 m−3 in a continuously powered plasma. Clearly large electron loss occurs in power-off phase for the first 10% (1 µs) of the duty cycle.
Figure 4. Electron density (continuous phase and volume averaged) 1 m−3 for a range of duty cycles over two modulation periods: Vapplied = 325 V, O2 admixture = 0.6%, modulation frequency 100 kHz.
Download figure:
Standard image High-resolution imageThe balance of diffusive, migrative and collisional forces determines the rate of charged species decay. The rate of electron loss is given by


Electron number density ne, flux Γe, production/loss term Qe. Migrative and diffusional losses of electron density are given by ∇ . Γe m−3 s−1. Collisional losses due to electron attachment (R17 in table 1) and ion–electron recombination (R14) reactions and gains by detachment (R24,25) reactions are given by Qe m−3 s−1. Migrative and diffusional losses of charged species in the absence of an external electric field are constrained by charge neutrality. Electron mobility is typically ∼100 times that of ionic mobilities [23] due to their mass differential. This mobility differential leads to the emergence of an electric field known as the ambipolar electric field which accelerates ion free diffusion and retards electron free diffusion. If we consider positive and negative ion mobilities are approximately equal (μp ≈ μn ≈ μi and Dp ≈ Dn ≈ Di ≈ μiTi) an effective migrative and diffusional electron loss term
 is given by the following [17]
is given by the following [17]


Here ne, np and nn are the electron, positive and negative ion density. When the electro-negativity (nn/ne) is small neμe ≫ μi(np + nn) with
 ≈ μi(Te + Ti). For high electro-negativity neμe ≪ μi(np + nn),
≈ μi(Te + Ti). For high electro-negativity neμe ≪ μi(np + nn),
 . For Te ≫ Ti the ratio
. For Te ≫ Ti the ratio
 [17]. Electron diffusion in electronegative portions of the plasma where Te ≫ Ti has therefore significantly higher rates than in electron-positive portions.
[17]. Electron diffusion in electronegative portions of the plasma where Te ≫ Ti has therefore significantly higher rates than in electron-positive portions.
Components of electron loss due to collisional and migrative-diffusive forces are shown in figure 5(b) in the power-off phase. Migrative-diffusive losses dominate initially with peak values of 4.1 × 1023 m−3 s−1 occurring at 6.02 µs. After 1 µs collisional and migrative-diffusive losses become comparable. This trend continues throughout the rest of the decay period with electro-negativity tending towards a steady state. Figure 5(b) shows the total electron loss rate (right axis) m−3 s−1 in the power-off period (6–10 µs) for a 60% duty cycle. Electron loss rates show a peak value of 4.3 × 1023 m−3 s−1 occurring at 6.05 µs. Electron losses in the first 0.1 µs, 0.5 µs and 1 µs of the 4 µs power-off phase account for 15.1%, 71.1% and 80.9% respectively of the overall electron loss. The majority of electrons are lost here in the first 0.5 µs. This initial decay period sets a limit for interference between power pulses which significantly shortens the initial growth period in electron density. Figure 5(b) shows the average electro-negativity in the power-off period (6–10 µs). The first ∼1 µs of the plasma decay phase (6–7 µs) results in a large increase in electro-negativity with average (volume) values increasing from 1.6 to 6.3 between 6 and 7 µs. This is due to electron attachment reaction R17 in table 1.
Figure 5. (a) Collisional and diffusive-migrative average electron loss rate m−3 s−1 in the power-off period (6–10 µs) for a 60% duty cycle (b) average electro-negativity (left axis) and electron loss rate (right axis) m−3 s−1 in the power-off period (6–10 µs) for a 60% duty cycle.
Download figure:
Standard image High-resolution imageFigure 6 shows the spatial distribution of charged species across the discharge gap at the start of the power-off period (6 µs) (figure 6(a)). The electric field across the domain is shown in figure 7. At t = 6 µs a sheath in the left-hand domain results in electron movement from the right-hand side to preserve quasi-neutrality. Figure 8 shows the effective electron diffusion coefficient
 (equation (4)) across the discharge gap. At t = 6 µs peak effective diffusion coefficients occur near x = −0.5 mm with values of ∼0.36 m2 s−1. The large initial electro-negativity in the left-hand side of the domain in the first 0.05 µs results in large electron loss
(equation (4)) across the discharge gap. At t = 6 µs peak effective diffusion coefficients occur near x = −0.5 mm with values of ∼0.36 m2 s−1. The large initial electro-negativity in the left-hand side of the domain in the first 0.05 µs results in large electron loss
 equation (4)) from the left-hand side of the domain. After 0.2 µs we see in figure 6(b) that the electron density is concentrated in the centre of the discharge gap along with the other charged species. The plasma density is enclosed between sheath regions of ∼0.2 mm width here. Figure 7 at t = 6.5 µs shows an electric field which is equal and opposite in the sheath region characteristic of ambipolar diffusion.
equation (4)) from the left-hand side of the domain. After 0.2 µs we see in figure 6(b) that the electron density is concentrated in the centre of the discharge gap along with the other charged species. The plasma density is enclosed between sheath regions of ∼0.2 mm width here. Figure 7 at t = 6.5 µs shows an electric field which is equal and opposite in the sheath region characteristic of ambipolar diffusion.
Figure 6. Charged species density m−3 across discharge domain at (a) t = 6 µs and (b) t = 6.2 µs for a 60% duty cycle.
Download figure:
Standard image High-resolution imageFigure 7. Electric field E (kV cm−1) across discharge gap at various times in power-off phase for 60% duty cycle.
Download figure:
Standard image High-resolution imageFigure 8.
 (m2 s−1) across discharge gap at various times in power-off phase for 60% duty cycle.
(m2 s−1) across discharge gap at various times in power-off phase for 60% duty cycle.
Download figure:
Standard image High-resolution image3.2. Power modulated gas heating
The contribution from various plasma heating mechanisms to gas heating is shown in figure 9 for a duty cycle of 60%. The average (volume and phase) steady state gas heating by the plasma is 1.49 × 107 W m−3 (0.45 W) during the power pulse. In the steady state power phase (>3 µs) elastic collisional heating dominates for on average 56.7% of the heating. Positive ion heating (
 accounts for 34% of the heating. Negative ion heating and inelastic heating due to heavy species collisions make up the remainder with contributions of 6.9% and 2.4% respectively. In the power off period (>6 µs in figure 9) gas heating is dominated by inelastic heating due to heavy species collisions (ion recombination reactions in table 1) with values several orders of magnitude lower than steady state contributions. Figure 10 shows results for the continuous phase and volume averaged total gas heating (Qavg(t): see section 2) over a modulation cycle for a range of duty cycles. The gas heating behaves similarly to the power growth shown in figure 2.
accounts for 34% of the heating. Negative ion heating and inelastic heating due to heavy species collisions make up the remainder with contributions of 6.9% and 2.4% respectively. In the power off period (>6 µs in figure 9) gas heating is dominated by inelastic heating due to heavy species collisions (ion recombination reactions in table 1) with values several orders of magnitude lower than steady state contributions. Figure 10 shows results for the continuous phase and volume averaged total gas heating (Qavg(t): see section 2) over a modulation cycle for a range of duty cycles. The gas heating behaves similarly to the power growth shown in figure 2.
Figure 9. Gas heating contributions (W m−3) over a modulation period: Vapplied = 325 V, O2 admixture = 0.6%, modulation frequency 100 kHz, 60% duty cycle.
Download figure:
Standard image High-resolution imageFigure 10. Total gas heating Qavg(t) (W m−3) over a modulation period for a range of duty cycles: Vapplied = 325 V, O2 admixture = 0.6%, modulation frequency 100 kHz.
Download figure:
Standard image High-resolution imageSolutions of a 2D gas mixing and heating model (see section 2) are given in figure 11, which shows the steady state surface temperature on a heat insulated treatment surface (BC in figure 1) 10 mm below the device for a range of duty cycles. Peak surface temperatures are contained here within a radius of 25 mm from the centre. The gas temperature at the surface is reduced from a peak value of 44 °C at 100% duty cycle to 23 °C at 20% duty cycle. At 5 mm device to surface separation a similar range of steady state surface temperatures of 23 °C to 44 °C at 20% and 100% respectively are found. At a 15 mm separation a range of 22.9 °C to 43.2 °C is found. Increasing the device to surface separation in this range results in quite a small decrease in peak gas temperatures at the treatment surface.
Figure 11. Gas temperature (°C) at an insulated treatment surface (BC in figure 1) at 10 mm below the device for a range of duty cycles: gas flow = 1.0 slpm (inlet at HI in figure 1), Vapplied = 325 V, O2 admixture = 0.6%, modulation frequency 100 kHz.
Download figure:
Standard image High-resolution imageThe effects of convective cooling on the gas temperature on a treatment surface (BC in figure 1) are shown figure 12. Steady state surface temperatures for volumetric flow rates of 1–2.5 slpm are shown here. Peak surface temperatures (x = 0) in the range of 44.6 °C–35.8 °C are found for increasing gas flow. The spatial behaviour of the surface temperatures shown in figure 12 shows larger volumes of heated gas impacting an increasing surface area at higher flows at lower temperatures. At 40 mm from the centre the temperature rises from 20 °C at 1 slpm to 35 °C at 2.5 slpm as greater volumes of heated helium displace colder ambient around the device. A comparison of power modulation and convection cooling is shown in figure 13. The duty cycle is varied for an inlet flow of 1 slpm here. Figure 13 demonstrates a significantly larger range of temperature control using power modulation than gas cooling by convection. Convection cooling shows a saturation behaviour at higher gas flows in comparison to duty cycle variation between 20–100% which gives linear control of surface temperatures. Lowering heat flux to a treatment surface at a prescribed distance without changing the gas flow may be advantageous in some application scenarios, for example in the use of atmospheric jets in clinical settings where patients find large gas flows uncomfortable or when large gas flows may undesirably disturb surface materials.
Figure 12. Gas temperature (°C) at an insulated treatment surface (BC in figure 1) at 10 mm below the device for a range of volumetric flow rates (inlet at HI in figure 1), Vapplied = 325 V, O2 admixture = 0.6%, modulation frequency 100 kHz for a 100% duty cycle.
Download figure:
Standard image High-resolution imageFigure 13. Comparison of steady state surface temperature (K) over an insulated surface (BC in figure 1) at 10 mm below the device for variation in duty cycle (at 1 slpm) and volumetric flow rate (1–2.5 slpm) Vapplied = 325 V, O2 admixture = 0.6%, modulation frequency 100 kHz.
Download figure:
Standard image High-resolution image3.3. Power modulated reactive species behaviour
The continuous phase and volume averaged atomic oxygen production Ravg(t) (see section 2) are shown in figure 14 for a range of duty cycles. Atomic oxygen production follows the power growth here (see figure 2). This is due to the strong coupling of power to the electron density and energy which directly affect the dominant O production reactions (R12, R13 in table 1). Peak production values at a steady state power (>3 µs) of 6.4 × 1024 m−3 s−1 are shown in figure 14.
Figure 14. Atomic oxygen production Ravg(t) m−3 s−1 over a modulation period for a range of duty cycles: Vapplied = 325 V, O2 admixture = 0.6%, modulation frequency 100 kHz.
Download figure:
Standard image High-resolution image2D solutions from a reacting and mixing gas model of neutral (uncharged) plasma produced reactive species (see section 2) with a fixed gas temperature of 300 K are shown for O density (ppm) in figure 15 on a treatment surface (BC in figure 1) for a range of duty cycles. Peak O values are contained here within a radius of 20–25 mm from the centre corresponding to the device width (KL-GF in figure 1). Peak O density of 19 ppm (4.9 × 1020 1 m−3) occurs in the centre (x ∼ 0) for 100% duty cycle with values of 17.9, 15, 11.3 and 6.3 ppm for 80, 60, 40 and 20% duty cycle respectively. The change between 40–100% shows a similar increment of ∼3 ppm per 20% increase in duty cycle. The larger O increase from 20–40% (∼5 ppm) is due to the operation at a steady state power (>30%) for a portion of the duty cycle. At a smaller device to surface separation of 5 mm, surface O density is found to range from 15 to 49 ppm for 20–100% duty cycle variation. Limited conversion of atomic oxygen to ozone via O2 (O + O2 → O3 + He (see R2 in table II in [18])) results in a larger O density at the treated surface here. This is a result of lower O2 concentration in the gas mixture and reduced residence time for O conversion to O3 at smaller separations. For a larger device separation of 15 mm a range of 1.7 to 7.8 ppm for 20–100% duty cycle variation is found consistent with higher O losses due to increased O2 and reaction time at higher device to surface separations.
Figure 15. O density at surface (BC in figure 1) at 10 mm below the device for variation in duty cycle: gas flow = 1 slpm, Vapplied = 325 V, O2 admixture = 0.6%, modulation frequency 100 kHz.
Download figure:
Standard image High-resolution imageSurface plots for O2(a 1Δ) and O3 density for a steady state solution of the 2D reacting and mixing gas model are shown in figures 16 and 17. O2(a 1Δ) values at the central peak range from 127 ppm (3.1 × 1021 1 m−3) at 100% duty cycle, 100 ppm at 80%, 75 ppm at 60%, 49 ppm at 40% and 22 ppm at 20%. O3 values at the central peak range from 270 ppm (6.6 × 1021 1/m3) at 100% duty cycle, 234 ppm at 80%, 195 ppm at 60%, 143 ppm at 40% and 74 ppm at 20%. At smaller separations between the device and surface of 5 mm; surface O2(a 1Δ) ranges from 155–24 ppm while O3 ranges from 258–67 ppm for 100%–20% duty cycle variation. At a 15 mm separation surface O2(a 1Δ) ranges from 107–19 ppm and O3 ranges from 268–73 ppm for 100%–20% duty cycle variation. The increase of O2(a 1Δ) at 5 mm and decrease at 15 mm is due to its interaction with O3 (O2(a 1Δ) + O3 → O + 2O2 (see R11 in table II in [18])).
Figure 16. O2(a 1Δ) density at surface (BC in figure 1) at 10 mm below the device for variation in duty cycle: gas flow = 1 slpm, Vapplied = 325 V, O2 admixture = 0.6%, modulation frequency 100 kHz.
Download figure:
Standard image High-resolution imageFigure 17. O3 density at surface (BC in figure 1) at 10 mm below the device for variation in duty cycle: gas flow = 1 slpm, Vapplied = 325 V, O2 admixture = 0.6%, modulation frequency 100 kHz.
Download figure:
Standard image High-resolution image4. Conclusion
Power modulation effects on plasma growth and decay, reactive species generation and gas heating are discussed for a cold atmospheric plasma jet. Power modulation is demonstrated as an effective mechanism for control of reactive species and heat flux delivery to a surface with interest towards applications in biomedicine and heat sensitive surface engineering. Power is shown to be coupled primarily to the electrons with electron loss rates determining the interference between successive modulation phases (10 µs). Plasma decay is characterized by a large initial electron loss in the first 0.5 µs followed by ambipolar decay dominated by ions of opposite charge for the remainder of the power-off period. This initial decay period sets a limit for interference between power pulses which significantly shortens the initial growth period in electron density. Power growth consists of an initial phase of exponential growth before transition to asymptotic convergence to a steady state value (∼2–3 µs). Power modulation effects on gas heating are shown to limit heat more effectively than cooling by convection at a treated surface. Surface densities of O, O2(a 1Δ) and O3 ROS on a treatment surface 10 mm below the device are found to typically range over an order of magnitude for variation in the duty cycle above 20% (2 µs). O, O2(a 1Δ) and O3 values reported here are consistent with previous experimental [7, 8, 31, 32] and numerical [13, 16, 26, 30, 31, 38, 48] studies of the µAPPJ conducted for a continuously powered discharge (100% duty cycle). A comparison of the numerical results presented here for atomic oxygen and ozone density for a continuously powered jet (100% duty cycle) with molecular beam mass spectrometry [8] is discussed in [19]. Further experimental and numerical investigation of pulsed powered jets is however clearly required to further understand and validate the qualitative and quantitative trends discussed in this report.
Acknowledgments
This material is based upon work supported by Science Foundation Ireland under Grant No 08/SRC/1411. The authors wish to acknowledge the SFI/HEA Irish Centre for High-End Computing (ICHEC) for the provision of computational facilities and support.